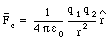
Ordinary matter consists of atoms. Each atom consists of a nucleus, consisting of protons and neutrons, surrounded by a number of electrons. The masses of the electrons, protons and neutrons are listed in Table 22.1. Most of the mass of the atom is due to the mass of the nucleus.
particle
|
mass
(kg)
|
| electron
|
9.11
x 10-31
|
| proton
|
1.673
x 10-27
|
| neutron
|
1.675
x 10-27
|
Experiments have shown that the electric force between two objects is proportional to the inverse square of the distance between the two objects. The electric force between two electrons is the same as the electric force between two protons when they are placed as the same distance. This implies that the electric force does not depend on the mass of the particle. Instead, it depends on a new quantity: the electric charge. The unit of electric charge q is the Coulomb (C). The electric charge can be negative, zero, or positive. Per definition, the electric charge on a glass rod rubbed with silk is positive. The electric charge of electrons, protons and neutrons are listed in Table 22.2. Detailed measurements have shown that the magnitude of the charge of the proton is exactly equal to the magnitude of the charge of the electron. Since atoms are neutral, the number of electrons must be equal to the number of protons.
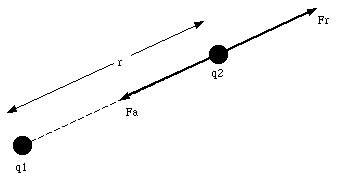
The precise magnitude of the electric force that a charged particle exerts on another is given by Coulomb's law:
" The magnitude of the electric force that a particle exerts on another particle is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. The direction of the force is along the line joining the particles. "
particle
|
charge
(C)
|
| electron
|
-
1.6 x 10-19
|
| proton
|
1.6
x 10-19
|
| neutron
|
0
|
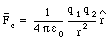
where
q1 and q2 are the charges of particle 1 and particle 2, respectively
r is the distance between particle 1 and particle 2 (see Figure 22.1)
[epsilon]0 is he permittivity constant: [epsilon]0 = 8.85 x 10-12 C2/(N . m2)
This formula applies to elementary particles and small charged objects as long as their sizes are much less than the distance between them.
Our discussion of the electric force will initially concentrate on those cases in which the charges are at rest or are moving very slowly. The electric force exerted under these circumstances is called the electrostatic force. If the charges are moving with a uniform velocity, they will experience both the electrostatic force and a magnetic force. The combined electrostatic and magnetic force is called the electromagnetic force.
particle-particle
|
Fg
(N)
|
Fc
(N)
|
| electron
- electron
|
-5.5
x 10-51
|
2.3
x 10-8
|
| electron
- proton
|
-1.0
x 10-47
|
-
2.3 x 10-8
|
| electron
- neutron
|
-1.0
x 10-47
|
0
|
| proton
- proton
|
-
1.9 x 10-44
|
2.3
x 10-8
|
| proton
- neutron
|
-
1.9 x 10-44
|
0
|
| neutron
- neutron
|
-
1.9 x 10-44
|
0
|
An important experiment in which the charge of small oil droplets was determined was carried out by Millikan (details of this experiment will be discussed in Chapter 23). Millikan discovered that the charge on the oil droplets was always a multiple of the charge of the electron (e, the fundamental charge). For example, he observed droplets with a charge equal to +/- e, +/- 2 e, +/- 3 e, etc., but never droplets with a charge equal to +/- 1.45 e, +/- 2.28 e, etc. The experiments strongly suggested that charge is quantized.
Another important property of charge is that charge a conserved quantity. No reaction has ever been found that creates or destroys charge. For example, the annihilation of an electron and an anti electron (positron) produces two photons:
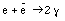
This reaction does not violate conservation of charge. The initial charge is equal to
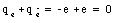
Note that the charge of an antiparticle is opposite that of the particle. The final charge is equal to zero since photons are uncharged. The following reaction however violates conservation of electric charge
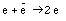
This reaction has never been observed.
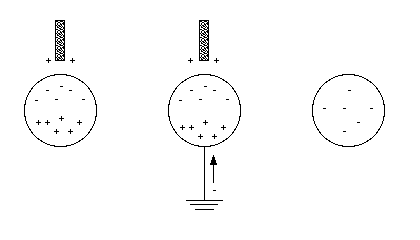
A conductor is a material that permits the motion of electric charge through its volume. Examples of conductors are copper, aluminum and iron. An electric charge placed on the end of a conductor will spread out over the entire conductor until an equilibrium distribution is established. In contrast, electric charge placed on an insulator stays in place: an insulator (like glass, rubber and Mylar) does not permit the motion of electric charge.