19. Entropy and the Second Law of Thermodynamics
19.1. Second Law of Thermodynamics
An engine changes heat into work. In the engine of a car the heat is generated by the combustion of an air-gas mixture. The heat is used to expand the gas, and lift the piston. Although work can be completely changed into heat, the reverse is not true (second law of thermodynamics). The first form of the second law is:
" It is not possible to completely change heat into work with no other change taking place "
Let us look at an ideal gas confined by a piston in a volume Vi. The gas is in contact with a heat reservoir with temperature T. The gas is permitted to expand by removing some weight of the piston. Its volume will increase while its temperature remains constant. Since the internal energy of the gas only depends on its temperature, the internal energy of the gas will not change. From the first law of thermodynamics we conclude that
![]()
This shows that we have converted all extracted heat into work. However, in the process we have also changed the state of the gas. Its volume and pressure have changed. In order to return the gas to its internal state we need to do work and extract heat from the system. In that case, the net work and heat will be zero.
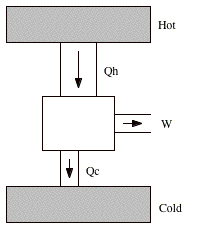
19.2. The Engine
The basic operation cycles of an engine consist of extracting heat from a reservoir, performing work, and supplying heat to another reservoir (see Figure 19.1). After one complete cycle the gas in the system returns to its original state. Since it has the same temperature at the beginning and at the end of the cycle, its internal energy did not change. The first law of thermodynamics tells us that the net work done per cycle therefore must equal the net heat transferred per cycle:
![]()
A measure of the performance of an engine is its thermal efficiency e, which is defined as the ratio of the work done per cycle and the heat absorbed per cycle
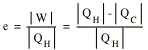
A 100% efficiency will be obtained if and only if QC = 0. However, the second law of thermodynamics clearly states that it is impossible to change heat completely into work. Thus, there are no perfect engines.
|
Figure 19.1. The engine. |
19.3. The refrigerator
A refrigerator is a device that causes heat to flow from a cold place to a warm place. Another formulation of the second law of thermodynamics states that
" It is not possible for heat to flow from one body to another body at a higher temperature
with no other change taking place "
The performance of a refrigerator is specified by the coefficient of performance K
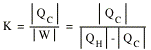
19.4. The Carnot Cycle
An example of a series of processes which would create a reversible engine is the carnot cycle. One full cycle consists of two isothermal and two adiabatic processes. The initial situation is specified by a volume Va, a pressure pa, and a temperature Ta. The gas is confined in a cylinder by a piston which has weight on top.
Step 1: the system is in contact with a high temperature reservoir. Thus, Ta = TH. Part of the weight of the piston is removed. As a result the gas will expand; its volume will increase from Va to Vb and its pressure will decrease. During this process a total heat equal to QH is extracted from the reservoir. Since the temperature of the gas is kept constant at TH all the heat extracted from the reservoir is transformed into work
![]()
Step 2: the cylinder is removed from the high temperature reservoir and put into an insulating stand. Some more weight is removed from the piston. As a consequence, the gas will further expand, and since there is no heat transfer during the expansion this is adiabatic expansion. During the expansion the temperature drops to TC and the volume of the gas increases from Vb to Vc. In chapter 21 we derived the following relation between temperature and volume for adiabatic processes
![]()
Thus gives the following relation between the initial and final temperature and volume
![]()
Step 3: The cylinder is put in contact with a colder heat reservoir and weight is added to the piston. As a result, the gas is slowly compressed (and the volume is decreased from Vc to Vd). During this compression, heat is transferred from the gas to the colder reservoir. Since there is no change in the temperature of the gas, its internal energy will not change. Therefore, during the isothermal compression the heat transferred to the colder heat reservoir is equal to the work done on the gas
![]()
Step 4: the cylinder is put in an insulating stand and more weight is added to the piston. The gas is compressed and its volume is decreased from Vd to Va. The temperature of the gas increases from TC to TH. Since there is no transfer of heat during the compression, the process is adiabatic. This means that
![]()
Thus gives the following relation between the initial and final temperature and volume
![]()
This relation can be rewritten as
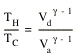
A similar relation can be obtained for the parameters involved in the adiabatic expansion of the gas
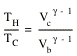
Combining these two equations for TH/TC we conclude that
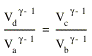
or
![]()
The heat extracted from the hot reservoir is given by
![]()
The heat delivered to the cold reservoir is given by
![]()
Thus,
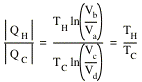
The efficiency of the cannot engine is therefore given by
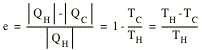
No real engine operating between two specified temperatures can have a greater efficiency than that of a carnot engine operating between the same two temperatures.
NOTE: SECTION ABOUT ENTROPY IS MISSING ! I DID NOT HAVE TIME TO FINISH IT ! BUT I ASSUME YOU TOOK GOOD NOTES DURING LECTURE ABOUT THIS CONCEPT !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!