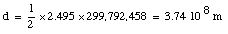
Measurements of physical quantities take place by means of a comparison with a standard. For example: a meter stick, a weight of 1 kilogram, etc.
The base units that will be used in this course are:
Example: how to measure the distance d from the earth to the moon ?
APOLLO astronauts placed a mirror on the moon. It can be used to measure the distance between the earth and the moon very accurately. The reflection of a laser beam aimed at this mirror reaches the earth after 2.495 s. The distance can then be calculated:
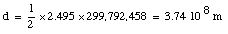
The definition of the standard mass makes it very inaccessible. In principle, the weight of individual nuclei can be used as a standard; nuclear weight does not depend on location, temperature, pressure, etc. However, counting the number of nuclei in a standard (or assembling a fixed number of nuclei) is an almost impossible task.
Note:
1. Improved definitions of base units must be defined such that it matches the previous definition as closely as possible (no need to change all meter sticks in 1983).
2. Speed of light is now defined as 299,792,458 m/s.
All physical quantities are expressed in terms of base units. For example, the velocity is usually given in units of m/s. All other units are derived units and may be expressed as a combination of base units. For example (see Appendix A):
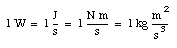
Other examples are:
When dealing with very small or large numbers, it is convenient to use prefixes (see Table 1.1, and Table 1-2 on page 3 in Halliday, Resnick, and Walker).
Factor
|
Prefix
|
Symbol
|
| 1018
|
exa-
|
E
|
| 1015
|
peta-
|
P
|
| 1012
|
tera
|
T
|
| 109
|
giga-
|
G
|
| 106
|
mega-
|
M
|
| 103
|
kilo-
|
k
|
| 10-3
|
milli-
|
m
|
| 10-6
|
micro-
|
u
|
| 10-9
|
nano-
|
n
|
| 10-12
|
pico-
|
p
|
| 10-15
|
femto-
|
f
|
| 10-18
|
atto-
|
a
|
Example: 2.35 10-9 s = 2.35 ns
In many cases, the data are not supplied in the correct units, and one needs to convert units (see Appendix F in Halliday, Resnick, and Walker).
Examples: 1" = 2.54 x 10-2 m
55 miles = 88 x 103 m
1 h = 3600 s
55 miles/h = 88 x 103/3600 m/s = 24.4 m/s