NOTE
This manual describes the laboratory experiment used during the 1996 - 1997 academic year. Significant changes have been made since then, and the manual used during the current academic year is in NOT available yet on the WEB. Hardcopies can be purchased at the bookstore.
To verify that atomic systems have discrete energy levels by bombarding electrons and observing the difference in energy levels with an electrometer.
The Bohr model of the hydrogen atom was successful in that
it correctly explained the spectrum of radiation from these atoms
to high accuracy. The model, in essence, indicated the presence
of discrete electronic energy levels in atoms, which could be
computed on the hypothesis that the angular momentum of the orbiting
electrons was quantized. A combination of the concept of quantized
energy levels with the idea that a quanta of radiation can only
be emitted (with frequency given by the Einstein relation ![]() ) when an atom changes its energy to a more
tightly bound state, completed the explanation of the spectrum
of atomic hydrogen. There are, however, other ways to get more
direct evidence of the existence of discrete energy levels in
atomic systems.
) when an atom changes its energy to a more
tightly bound state, completed the explanation of the spectrum
of atomic hydrogen. There are, however, other ways to get more
direct evidence of the existence of discrete energy levels in
atomic systems.
In this experiment we shall examine the effect of bombarding atoms of mercury with electrons. Since atoms are believed to have discrete energy levels one would expect that in collisions with electrons, the transfer of energy to the atom should occur in discrete amounts. One possible mechanism would be inelastic scattering in which a discrete amount of the incident electron energy is absorbed by the whole atom which is thus raised to an excited state. The kinematics of such a collision allow that almost all the energy of the incident electron can be absorbed by the atomic system, provided the atom does not become ionized.
Franck and Hertz first attempted to verify these ideas in 1914. Namely that the atomic systems have discrete energy levels which can be excited by collisions with bombarding electrons and that the energy differences in the levels correspond with the spectroscopic results. We shall repeat the Franck-Hertz experiment using mercury vapor. The spectral line observed in mercury discharges, which corresponds to the energy levels we shall observe, has a wavelength of 2537Å. Of course, there are many other lines in the mercury spectrum but it is extremely difficult to excite these energy differences in the mercury atom with a Franck-Hertz set-up.
The prelab homework must be done at home and handed to the TA before you start the lab.
A diagram of equipment to be used is shown in Figure 1. It consists of a thermionic tube with a cathode, an anode and a third electrode which is called the collector or counter electrode. The tube also contains a small blob of mercury. The metal casing surrounding the tube is grounded. This mercury is heated to 180deg.C to maintain a mercury vapor pressure in the tube. The pressure is, of course, temperature dependent and the amount of pressure is critical to the experiment. If the vapor density is too low, there may be too few mercury atoms to produce observable effects. At too high a pressure the reduction in the mean free path and thermal agitation becomes troublesome.
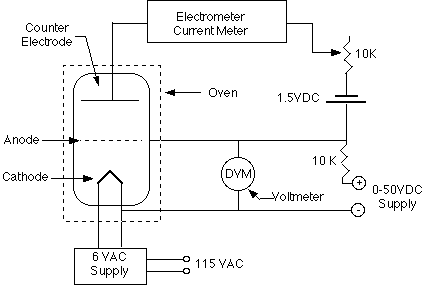
Figure 1
The anode is connected to a variable voltage source (0 to +50 Volts) with respect to the cathode. The counter electrode is maintained at 0.5 - 1.5 Volts negative with respect to the anode. The counter electrode voltage can be adjusted by a voltage divider, and a 1.5 volt battery.
The cathode-anode system acts basically as a diode. Some of the electrons can pass through the anode, a grid-like structure, and can, if energetic enough, overcome the 0.5 - 1.5 Volts retarding potential to hit the counter electrode. This counter current can be measured using the Keithley Electrometer which is a very sensitive device suitable for measuring the small current involved.
We must, however, consider how the presence of the mercury vapor modifies the behavior of the system. Electrons accelerated from the cathode to the anode will collide with the mercury atoms. When the electrons acquire sufficient energy to inelastically excite a mercury atom, the electron can lose most of its energy. It then may have insufficient energy to overcome the 0.5 - 1.5 Volts required of it to reach the counter electrode. Therefore, as one increases the anode voltage, one should see a sharp dip in the counter current at the energy at which such collisions can occur. At higher voltages the counter current starts to rise again but eventually the electrons gain sufficient energy to suffer two or more inelastic collisions. Thus, the observed current-voltage curve should be steadily rising with a superimposed series of dips; the separation of two dips will correspond to the difference in energy of the ground state and first excited state in the mercury atoms.
1. Melissinos, Experiments in Modern Physics. A good description of this experiment and its interpretation.
2. Richtmyer, Kennard and Lauritsen, Introduction to Modern Physics, p. 161ff. General Discussion.
3. Harnwell and Livingood, Experimental Atomic Physics, pp. 314-323. Good on experimental details.
4. Hoag and Korff, Electron and Nuclear Physics, Ch. 7, pp. 151-158. General discussion plus experiment.
5. Fano and Fano, Basic Physics of Atoms and Molecules, p. 40-43. General.
6. Taylor, Advanced Undergraduate Experiments in Physics, pp. 410-412. Experimental set-up.
7. Peaslee, Elements of Atomic Physics, p. 158 ff. More discussion of F-H.
8. Leybold Data Sheets.
9. Franck and Hertz, Verhand. Deut. Physik Ges., 16, 512, (1914). The original experiment.
10. Foote, Meggers and Mohler, Astrophysical Journal, 55, 145, (1922). Excitation of sodium and potassium vapor studied.