![]()
Exponential decay occurs whenever a quantity, call it Q(t), changes at a rate,
![]()
proportional to the value of Q(t) itself. This equation can be integrated (this is an exercise for the reader) to obtain,
![]()
where
![]() = Q(0), the value of Q at t = 0. This characteristic functional
evolution accounts for the name: exponential decay.
= Q(0), the value of Q at t = 0. This characteristic functional
evolution accounts for the name: exponential decay.
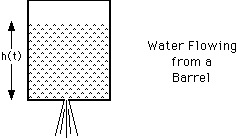
For example, suppose there is a barrel of water with a small hole on the bottom
as shown in the figure below. Water will flow out of the hole at a rate
proportional to the pressure P of the water at the bottom of barrel. If
Q is the volume of water in the barrel,
![]() where the constant k depends on things like the size of the
hole. Now
where the constant k depends on things like the size of the
hole. Now
![]() ,
where r is the density of water, g is the acceleration of gravity and
h(t) is the height of the water at time t. The volume of the water is
,
where r is the density of water, g is the acceleration of gravity and
h(t) is the height of the water at time t. The volume of the water is
![]() ,
where A is the area of the base of the barrel. Therefore, the pressure
,
where A is the area of the base of the barrel. Therefore, the pressure
![]() is proportional to Q(t) and so,
is proportional to Q(t) and so,
![]()
![]()
where Q0 is the volume of water in the barrel at time t = 0 and
![]() .
.
It was convenient to write the proportionality constant as 1/t because it is the reciprocal of the constant of proportionality, t, called the time constant or lifetime, which has direct physical significance.
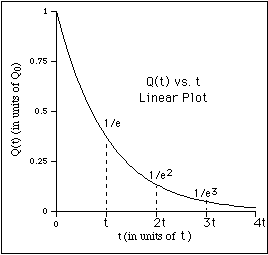
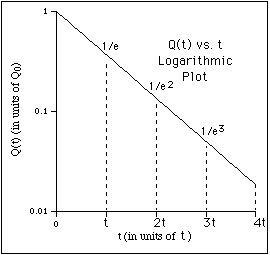
In the case of the water barrel, the lifetime is the time it takes for the water (volume) to drop by a factor of
1/e (~ 0.37). This can be seen by evaluating Q(t) at t = t to obtain,
![]() .
(5)
.
(5)
Another useful quantity is called the half-life T1/2. Again, in the case of the water barrel, this is the time it takes the volume of the water to drop to half of what it was at t=0; that is,
![]()
![]()
so
![]()
gives the relation between the time constant and the half-life.
A classic example, where the concepts of lifetime and half-life are commonly used, is the description of radioactive decay. In this case, Q(t) is the number of atomic nuclei at given time. Since the probability of decay per nucleus is a constant, the rate of decrease of the nuclei is proportional to the number of nuclei and the result is exponential decay.
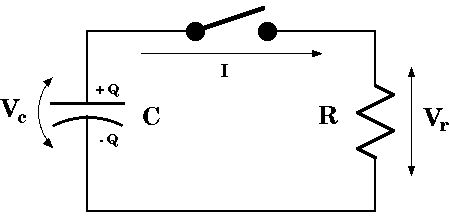
A resistor-capacitor circuit is another example. The voltage on a capacitor is
proportional to its charge,
![]() .
The voltage across a resistor is proportional to the current through it,
.
The voltage across a resistor is proportional to the current through it,
![]() .
If a resistor is connected across an initially charged capacitor (the switch
in the diagram is closed) then the charge will flow through the resistor,
.
If a resistor is connected across an initially charged capacitor (the switch
in the diagram is closed) then the charge will flow through the resistor,
![]() ,
and dissipate. Thus, since the voltages across the resistor and capacitor will
be equal,
,
and dissipate. Thus, since the voltages across the resistor and capacitor will
be equal,
![]()
This is just exponential decay with a time constant given by t = RC.
In the discussion of exponential decay, it was convenient to think of the independent variable as time. This is often the case, as it was in all the above examples, but the independent variable could be anything.
As a final example of exponential decay, in this instance for a variable that is not time, consider the earth's atmosphere. The pressure at a given height h is given by the perfect gas law
![]()
in terms of the density r(h) at that height and the average molecular mass M of the atmosphere. But the force of gravity on a small section of the atmosphere
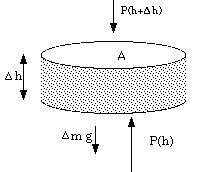
![]()
therefore,
![]()
or, in the limit,
![]()
This is making the (very poor, in this context!) assumption that the acceleration due to gravity does not depend on height. Combining this with the previous result gives,
![]()
the rate of change of the pressure with height to be proportional to itself. Thus, the pressure of the atmosphere decays exponentially with height as
![]()
where
![]() is the distance in which the atmospheric pressure decreases by a factor of
e and
is the distance in which the atmospheric pressure decreases by a factor of
e and
![]() =
P(0) is the pressure at h = 0 (say, ground level).
=
P(0) is the pressure at h = 0 (say, ground level).