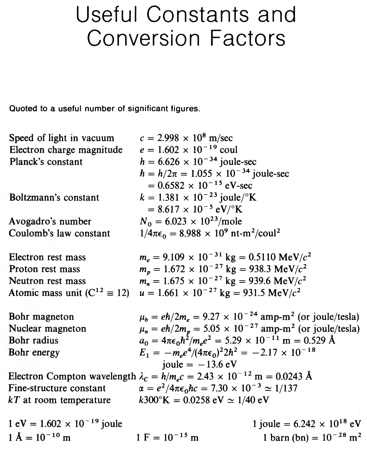
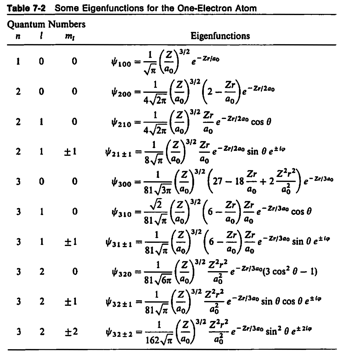
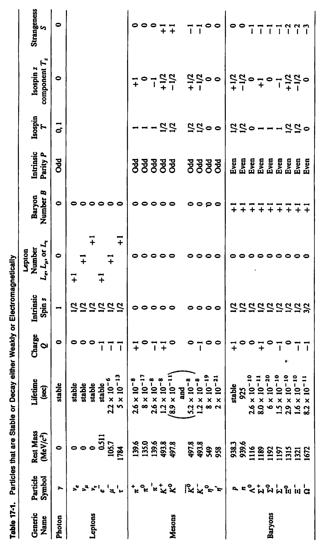
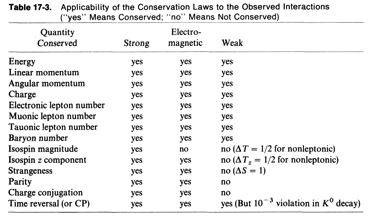
Physics 237, Final Exam
Wednesday
May 5, 2010
7.15 pm
– 10.15 pm
Do not turn the pages of
the exam until you are instructed to do so.
Exam rules:
You may use only a writing instrument
and your equation sheet while taking this test. You may not
consult any calculators, computers, books, or each other.
1. Problems 1, 2, and 3 must be answered in booklet # 1.
2. Problems 4, 5, 6, and 7 must be answered in booklet # 2.
3. The answers need to be well motivated and expressed in terms of the variables used in the problem. You will receive partial credit where appropriate, but only when we can read your solution. Answers that are not motivated will not receive any credit, even if correct.
At the end of the exam,
you need to hand in your exam, your equation sheet, and the three blue exam
booklets. All items must be
clearly labeled with your name, your student ID number, and the day/time of
your workshop.
Name: __________________________________________________
ID number: ______________________________________________
Workshop Day/Time: ______________________________________
INTENTIONALLY LEFT BLANK
Problem 1 (35 points) ANSWER
IN BOOKLET 1
Consider the motion of an
electron of mass m under the
influence of the following potential V:
![]()
a)
What is the
general solution of the time-independent Schrdinger equation for this system?
b)
What is the
energy of the solution obtained in part a)?
c)
What is the
zero-point energy of the system?
d)
If at time t = 0, the electron is in a state
corresponding to the first excited state, what is the probability that the
electron will be in that same state at time t
= lC/c where lC is
the Compton wavelength of the electron?
Problem 2 (30 points) ANSWER
IN BOOKLET 1
Consider
one electron in the n = 2 shell of a
one-electron atom. If we ignore
the spin-orbit coupling, then the wavefunctions in the n = 2 shell are degenerate.
Assume that the electron can be found with equal probability in each of
the degenerate n = 2 states.
a)
Write down the
wavefunction describing this electron in terms of the eigenfunctions of the
one-electron atom. Make sure your
wavefunction is properly normalized.
b)
What is the
shape of the probability density distribution of this electron? You will need to specify the r dependence, the q dependence, and the f dependence of the probability density distribution.
Problem 3 (35 points) ANSWER
IN BOOKLET 1
Consider
a He atom. When He is in its
ground state, both electrons are in the 1s
state.
a)
What is the
proper spectroscopic notation of the ground state of He?
If
we shine ultraviolet light on the He atoms, we can excite the atom into its
first few excited states. These excited
states have one electron in the 1s
state and one electron in the 2s or
in the 2p state.
b)
What is the
proper spectroscopic notation of the excited states in He when the electrons
are in a (1s)(2s) configuration?
c)
What is the
proper spectroscopic notation of the excited states in He when the electrons
are in a (1s)(2p) configuration?
d)
Draw an
energy-level diagram, showing all the states of He discussed in parts a), b),
and c). Label each state with the
proper spectroscopic notation. Do not ignore the spin-orbit
coupling.
e)
In the diagram
obtained in part d), indicate which transitions can occur between the excited states
discussed in parts b) and c) and the ground state discussed in part a).
f)
If we put the
atom in an external magnetic field of strength B, we see an increase in the number of transitions we can
observe. How many transitions will
we be able to observe now?
Problem 4 (30 points) ANSWER
IN BOOKLET 2
Consider a photon, moving in one
dimension in a region where V = 0.
a)
Starting with
the relativistic expression for total energy in terms of linear momentum and
mass, formulate the Schrdinger equation for the photon.
b)
Use separation
of variables to solve the Schrdinger equation. What is the wavefunction that describes the photon?
Problem 5 (30 points) ANSWER
IN BOOKLET 2
An energy diagram for Hydrogen is
shown in the Figure below.
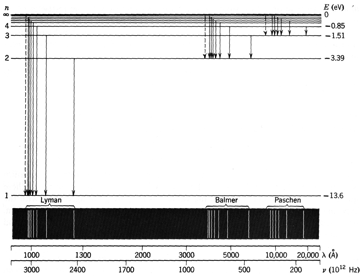
Two techniques can be used to confirm
the energies of the levels of Hydrogen: studies of emission spectra and studies
of absorption spectra.
At low temperatures, the absorption
spectrum is dominated by transitions that are part of the Lyman series. Estimate the temperature at which
Balmer lines will be observed in the absorption spectrum. Note: you do not need to evaluate the numerical expression you obtain for
the temperature T.
Problem 6 (35 points) ANSWER
IN BOOKLET 2
a)
Consider the
following energy-level diagram for atoms with 14 nucleons. Energy levels with similar properties
are connected with dashed lines.
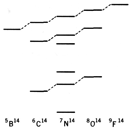
Identify
the isospin and the z component of
the isospin for all states shown in the diagram.
b)
Consider the
following reactions:
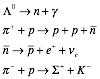
For each
of these reactions, state the fastest interaction through which it can
proceed. If the reaction is
forbidden, indicate why.
c)
Consider the 4
lowest energy levels observed for the two-nucleon system, shown in the Figure
below.
![]()
For each
of the four states shown in the Figure, determine the total spin. Which of these states are stable? What is the cause of the energy
difference between the state shown for 2He and the state shown for 0n?
Problem 7 (5 points) ANSWER
IN BOOKLET 2
Match the following players
a)
Y Berra
b)
B Williams
c)
M Mantle
d)
D Jeter
e)
L Gehrig
to the following At Bat (AB) numbers
of these players for the New York Yankees:
___ 8757
___ 8102
___ 8001
___ 7869
___ 7546
Note: these number were correct on May
1, 2010 but may have changed on the day of the exam.
