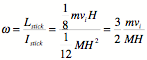Problem 1 (2.5 points)
|
1 |
2 |
3 |
4 |
Match the above shown players of the best
baseball team in the world with the following names:
A. Derek Jeter
B. Mariano Rivera
C. Johnny Damon
D. Jorge Posada
1234 =
a. ABCD
b. ACDB
c. BADC
d. BDAC
e. CADB
f. CABD
g. DBAC
h. DCBA
Problem 2 (2.5 points)
As you hold the string, a yoyo is released from rest so that gravity pulls it down, unwinding the string. What is the angular acceleration of the yoyo, in terms of the string radius R, the moment of inertia I, and the mass M?
1. g/(R + 2I/(MR))
2. gMR/I
3. gMR/(I
+ MR2)
4. g/R
Problem 3 (2.5 points)
Suppose you are holding a bicycle wheel by a handle, connected to the axle, in front of you. The axle points horizontally away from you and the wheel is spinning clockwise from your perspective. You now try to tilt the axle to your left (center of mass moves leftward). The wheel will swerve
1. upward.
2. downward.
3. to your left.
4. to your right.
Problem 4 (2.5 points)
An ideal gas is contained in a small volume, which is connected to a much larger volume that contains a vacuum. Both volumes are insulated. When the valve between the two volumes is opened, the gas will expand until it fills both volumes. During this expansion, the gas
1. does positive work.
2. increases its internal energy.
3. decreases its internal energy.
4. does
not change its internal energy.
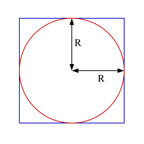 Problem 5 (2.5 points)
Problem 5 (2.5 points)
The moment of inertia of a square plate of area 4R2 and mass M, with respect to an axis through its center and perpendicular to the plate, is equal to (2/3)MR2. A disk of radius R is removed from the center of the plate (see Figure). What is the moment of inertia of the remaining material with respect to the same axis?
1. (p/8)MR2
2. (1/3 - p/12)MR2
3. (1/6)MR2
4. (2/3
- p/8)MR2
Problem 6 (2.5 points)
What is the heat capacity per atom or molecule in a solid
1. 3k
2. 3/2 k
3. 5/2 k
4. 7/2 k
Problem 7 (2.5 points)
What is the heat capacity at constant volume of a diatomic gas at a temperature T where kT is large compared to the energy of the first vibrational excited state?
1. 3k
2. 3/2 k
3. 5/2 k
4. 7/2
k
Problem 8 (2.5 points)
According to the Fundamental Assumption of
Statistical Mechanics, which of the following states of an atom with three
degrees of freedom and three quanta of energy is most probable?
1. One degree of freedom with 3 quanta of energy and two degrees of freedom with 0 quanta of energy each.
2. One
degree of freedom with 2 quanta of energy, one degree of freedom with 1 quantum
of energy, and one degree of freedom with 0 quanta of energy.
3. Three degrees of freedom with 1 quantum of energy each.
4. None of the above, because all microstates are equally probable.
Problem 9 (2.5 points)
Two wheels, initially at rest, roll the same
distance without slipping down identical inclined planes. Wheel B has twice the radius but the same mass as wheel A. All
the mass is concentrated in their rims, so that the rotational inertias are I = mR2.
Which wheel has the largest rotational kinetic energy when
it gets to the bottom?
1. Wheel A.
2. Wheel B.
3. The
rotational kinetic energies are the same.
4. Need more information.
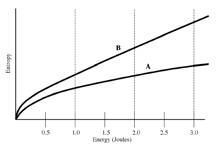 Problem
10 (2.5
points)
Problem
10 (2.5
points)
The figure on the right shows a plot of the entropy of two different metal blocks as a function of the internal (thermal) energy. Suppose the blocks (labeled A and B) are isolated from each other and are warmed until they each have 2 Joules of thermal energy. Which block has a lower temperature at this energy?
1. Block A has a lower temperature.
2. Block
B has a
lower temperature.
3. They have the same temperature since the thermal energy is the same.
4. There is not enough information to determine the temperature. The mass and specific heat of each block must be provided.
Problem 11 (25 points)
A cylinder with cross sectional area A contains N molecules of helium gas at pressure p0 and is in thermal equilibrium with a
heat bath of temperature T0. A piston confines the gas inside a
region of volume V0. The entire system is contained in a
vacuum vessel, and only the helium gas exerts a pressure on the piston. Assume that g = Cp/CV = 5/3 (for Helium).
a. You quickly pull up the piston to increase the volume of the gas to Vf. What is the temperature Tf of the gas immediately after you finish pulling up the piston? What approximations did you make?
Since the piston is pulled up quickly we can assume that this is an adiabatic expansion. During an adiabatic expansion p V g = constant. Using the ideal gas law we can rewrite this in terms of the temperature T and the volume V: T V g-1 = constant. Applying this relation to the expansion we conclude that
![]()
or
![]()
b. What is the work done by the gas during this expansion?
The work done by the gas during the expansion can be found by determining the area under the p versus V curve:
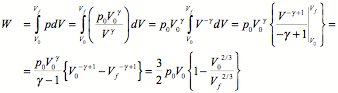
c. What is the force you must exert on the piston, immediately after you finish pulling it up, in order to hold it into its final position?
Since pVg = constant we can easily calculate the pressure of the gas right after the adiabatic expansion:
![]()
The force on the piston is thus equal to
![]()
Note: we could have also used the ideal gas law and the temperature calculated in part a) to determine the final pressure:
![]()
d. You wait until the helium returns back to its original temperature T0. What is now the force you must exert on the piston in order to hold it into its final position?
We can use the ideal gas law to determine the pressure of the gas when it has returned to its original temperature T0:
![]()
The force on the piston at this point is thus equal to
![]()
e. You now very slowly move the piston back to its original position such that the gas is contained in a volume V0. How much work must you do to move the piston back to this position? Is the magnitude of this work larger or smaller than the magnitude of the work calculated in part b? What approximations did you make?
The compression we now carry out is an isothermal compression. Since this compression is slow, there is ample of time to assure that the gas is maintained at a temperature T0. The work done by the gas, which is opposite to the work that you need to do, can be calculated using the ideal gas law:
![]()
The work calculate in part b can be rewritten as
![]()
To compare the work done by these two processes, we really compare the work done during an isothermal process with the work done during an adiabatic process. If we compare the area under the pV curve for an adiabatic process with the work done under the pV curve for an isothermal process with the same starting conditions (same p, same V) we see that the work done on the gas during an adiabatic compression is less than the work done by the gas during an isothermal expansion (see Figure below).
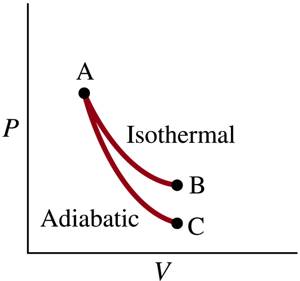
Problem 12 (25 points)
a. What
is the heat capacity per molecule at constant volume of a monatomic gas, such
as helium or neon. Why doesn’t the
heat capacity depend on temperature?
The heat capacity per molecule for a monatomic gas is 3/2k. A
monatomic gas only has 3 degrees of freedom (translational degrees of freedom)
and no rotational or vibrational states that can be excited. As a result, the number of degrees of
freedom and thus the heat capacity is not temperature dependent.
b. What
is the heat capacity per molecule at constant volume of a diatomic gas, such as
oxygen and nitrogen, at very high temperatures?
For a diatomic gas we need to consider the rotational and
vibrational states. At the highest
temperature the total number of degrees of freedom is 7, and the heat capacity
per molecule of the diatomic gas is 7/2k.
c. Suppose
we lower the temperature of a diatomic gas to a point where kT is small compared to the first excited rotational
state. What is the heat capacity
per molecule at constant volume of this gas at this temperature?
When we lower the temperature to a point where kT is small compared to the first excited rotational
state, the molecule will only have three degrees of freedom. As a result, the heat capacity per
molecule of the gas is 3/2k.
d. The
transition temperature Ttransition
is the temperature at which the rotational properties of the gas molecules need
to be taken into consideration when describing the properties of a gas. Consider the following two gases: H2 (hydrogen, whose nuclei contain a single
proton) and D2
(deuterium, whose nuclei contain a proton plus a neutron). Estimate the ratio of the transition
temperatures of these gases, Ttransition,
hydrogen/Ttransition,
deuterium. Your answer needs
to be well motivated and any approximations you have made must be clearly
stated.
The energy of rotational states scale with 1/I, where I
is the moment of inertia. The
lowest rotational state corresponds to a rotation around an axis that maximizes
I. The hydrogen and deuterium molecule have the same size (the
molecular size is determined by the inter-atomic force which depends only on
the electric force). The largest
moment of inertia is obtained when we consider a rotation around an axis perpendicular
to the line connecting the two atoms.
The moment of inertia is proportional to the atomic mass, and the moment
of inertia of deuterium is thus twice the moment of inertia of hydrogen: Ihydrogen /Ideuterium
= 1/2. The ratio of energies of the
lowest rotational excited states is equal to DErot,hydrogen/DErot,deuterium = 2. Since the energy of the molecules is proportional to T, we expect that the ratio of transition temperatures
scales as the ratio of energies: Ttransition,
hydrogen/Ttransition,
deuterium = 2.
e. Consider
two volumes of hydrogen and deuterium gas, both maintained at the same
temperature T. The temperature T
is low enough to ensure that the rotational and vibrational states are not
excited. Estimate the ratio of the
speed of sound in hydrogen and deuterium gas, vsound, hydrogen/vsound, deuterium. Your answer needs to be well motivated
and any approximations you have made must be clearly stated.
The speed of sound is proportional to the root-mean-square
speed of the molecules in the gas.
The rms speed of a gas molecule is equal to √(3kT/M). Since
both gases are maintained at the same temperature, their speed of sounds will
differ due to differences in their molecular mass. Since the mass of a deuterium molecule is twice the mass of
a hydrogen molecule, the rms speed of the deuterium molecules will be 1/√2
times the rms speed of the hydrogen molecules. The speed of sound in deuterium gas will thus be 1/√2 times
the speed of sound in hydrogen gas: vsound, hydrogen/vsound,
deuterium = √2.
Problem 13 (25 points)